Abstract
The two first authors contributed equally.
Introduction
There is currently little data on the outcome of AML in adolescents and young adults (AyAs) after allogeneic HSCT. In this retrospective study from the SFGM-TC registry, we analyzed the outcome of AML patients classified in 3 groups according to the age at transplantation: children (<15 years), AyAs (15-25 years) and adults (26-40 years) in the goal to determine factors influencing survival and NRM differences.
Patients and Method
This study included 2240 patients from 43 centers among France, Belgium, Switzerland, Lebanon and Algeria, of whom 481 children (21.5%), 545 AyAs (24.3%) and 1214 adults (54.2%). All patients received a first allogeneic HSCT for AML between 2005 and 2015. Five-years OS, NRM, GRFS and EFS and cumulative incidence of relapse were compared between the three groups of patients.
Results
The median follow-up of the study was 7 years (range, 4-9.6). According to the 2016 WHO classification, children had the highest rate (31.8%) of adverse risk disease, followed by AyAs (25.2%) and adults (21.3%) (p=0.0001). Extramedullary disease was observed in 42.8%, 24% and 21.4 % of children, AyAs and adults (p=0.0001). Among patients in complete remission (CR), younger age was associated more frequently with advanced disease (29.9% ≥ CR2 for children, 23% for AyAs and 18.2% for adults) (p=0.0001). Donors were matched siblings in 36.4% of children, 36.7% of AyAs and 40.3% of adults, and matched unrelated in 29.1%, 33.4% and 35.7% respectively. Children had more mismatched unrelated donors (31.6%), compared to AyAs (25.3%), and adults (19.5%) (p=0.0001). Stem cell source was different between the 3 groups (p=0.0001): bone marrow (BM) was the main source for children (60.9 % of grafts) followed by cord blood (CB) (28.9%), whereas peripheral blood stem cells (PBSC) was the main source for adults (60 %), followed by BM (29.7%) and CB (10.2%). AyAs were transplanted with BM in 44.4% of cases, PBSC in 40.4% or CB in 15.2%. The intensity of the conditioning was myeloablative (MAC) in 93% of children, 79% of AYAs and 77.7% of adults, while a reduced-intensity regimen was used in 4% of children, 12.3% of AyAs and 14.1 % of adults (p=0.0001).
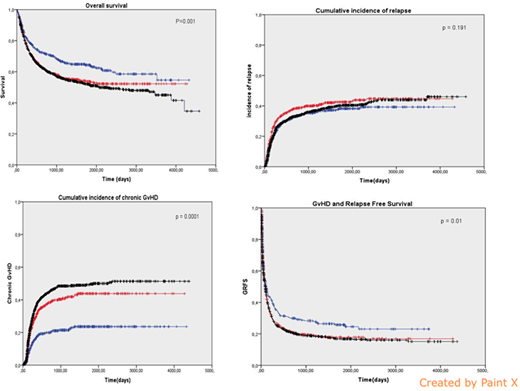
The 5y-overall survival (OS) was higher in children (64%), compared to AyAs (54%) and adults (52%) (p=0.001) (figure). One-year cumulative incidence (CI) of relapse was comparable in the 3 groups: 27 % for children, 33% for AYAs and 28% for adults (p=0.19) (figure). The 5y-CI of EFS and the 5y-CI of NRM were better for children (56%) compared to AyAs (49%) and adults (47%) (p=0.017) respectively and 11%, 18% and 27% for NRM in each group respectively (p=0.0001).
Regarding causes of deaths, GvHD related mortality was significantly higher in AyAs and adults with 3.1%, 5.1% and 7.7% in each group respectively. There was no difference in the CI of grade II-IV acute GvHD at day +100 between the 3 groups: 37.7%, 35.8% and 35% respectively (p=0.58) while the one-year CI of chronic GvHD was higher in adults (37%) and AyAs (31%) compared to children (17%) (p=0.0001) (Figure).
AyAs had a significantly lower 5y-OS and 5y-EFS than children and AyAs had a greater risk of NRM than children in this study. Moreover the GRFS is significantly better in children with 5y-survival 26% for children, 18% for AyAs and 17% for adults (p=0.011) (figure).
In univariate and multivariate analysis, independent factors associated with OS were age, TBI, cytogenetics and status of disease, HLA matching and donor age.
Conclusion
AyAs patients seem to have greater risk of NRM and cGvHD after HSCT than children. This study suggests that AyAs should be treated more like children and that donor age and HLA compatibility should be considered in the treatment strategy.
Chalandon:Roche: Membership on an entity's Board of Directors or advisory committees, Other: Travel costs. Beguin:Kiadis Pharma: Consultancy. Peffault De Latour:Amgen Inc.: Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Pfizer Inc.: Consultancy, Honoraria, Research Funding; Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.